Problem – The TMF Intake Bottleneck
Keeping the Trial Master File complete, accurate, and inspection-ready is critical—but doing it manually is slow, error-prone, and expensive. TMF teams are buried under:
Delays in filing, inconsistent quality, late discovery of issues, and constant inspection anxiety.
Intelligent Document Intake & Routing
Think of it as a dedicated digital TMF intake specialist that works 24/7, never gets tired, and documents everything it does.
Intelligent Document Intake & Routing
-
- Drag-and-drop upload or bulk import from email, portals, and shared drives
- Or Connect to document source such as shared folder, landing area or even eTMF system which is already deployed
- Automatically routes documents to the right TMF intake queue based on study, site, artifact type, and priority
- Flags urgent items (e.g., Urgent Project Document, serious safety documents) so nothing critical is missed
.png)
Intelligent Document Intake & Routing
Intelligent Document Intake & Routing
Guided Metadata Prompting at Upload
.png)
Guided Metadata Prompting at Upload
Guided Metadata Prompting at Upload
Ensure every document enters your TMF with the right context.
Mandatory metadata prompts:
-
- Study / Protocol
- Accountable Party
- Artifact Owner
- Document Received Externally (+ Date)
- Wet Ink (Yes/No)
- Urgent Project Document (Yes/No)
Optional metadata prompts:
-
- Study Country
- Study Site
- Description shared with site(s)
- Other Document Notes
The Agent combines AI suggestions + user prompts, reducing typing while enforcing completeness and consistency.
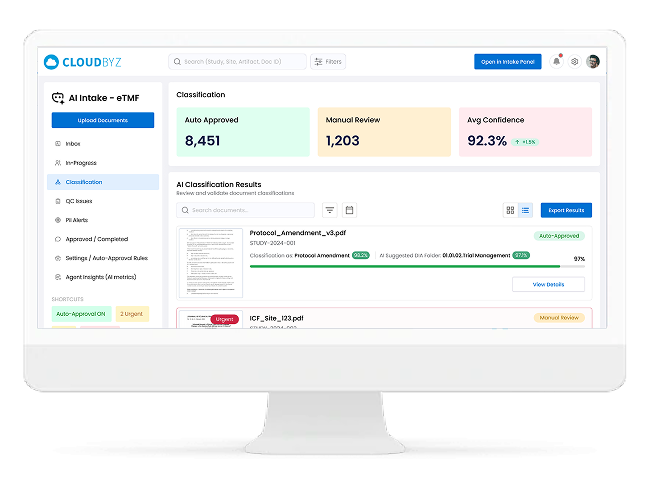
AI-Based Classification with Confidence Scores
-
- Classifies documents to the correct TMF artifact / zone / section using AI
- Displays clear confidence scores (e.g., 98%, 72%) so reviewers know when to trust vs. verify
- Suggests multiple candidate artifact types when confidence is low, speeding up human review

AI-Based Classification with Confidence Scores
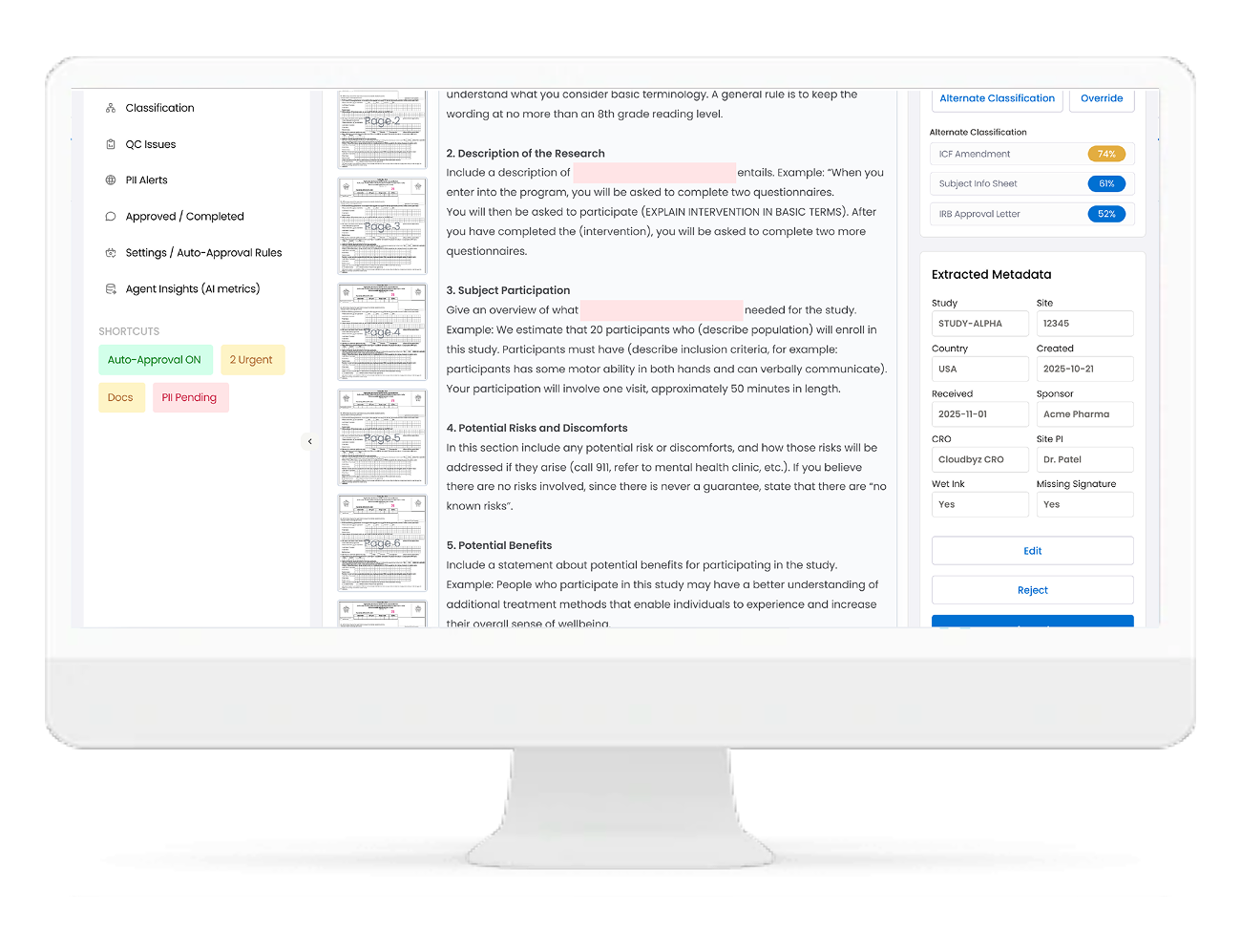
Automatic Metadata Extraction & Population
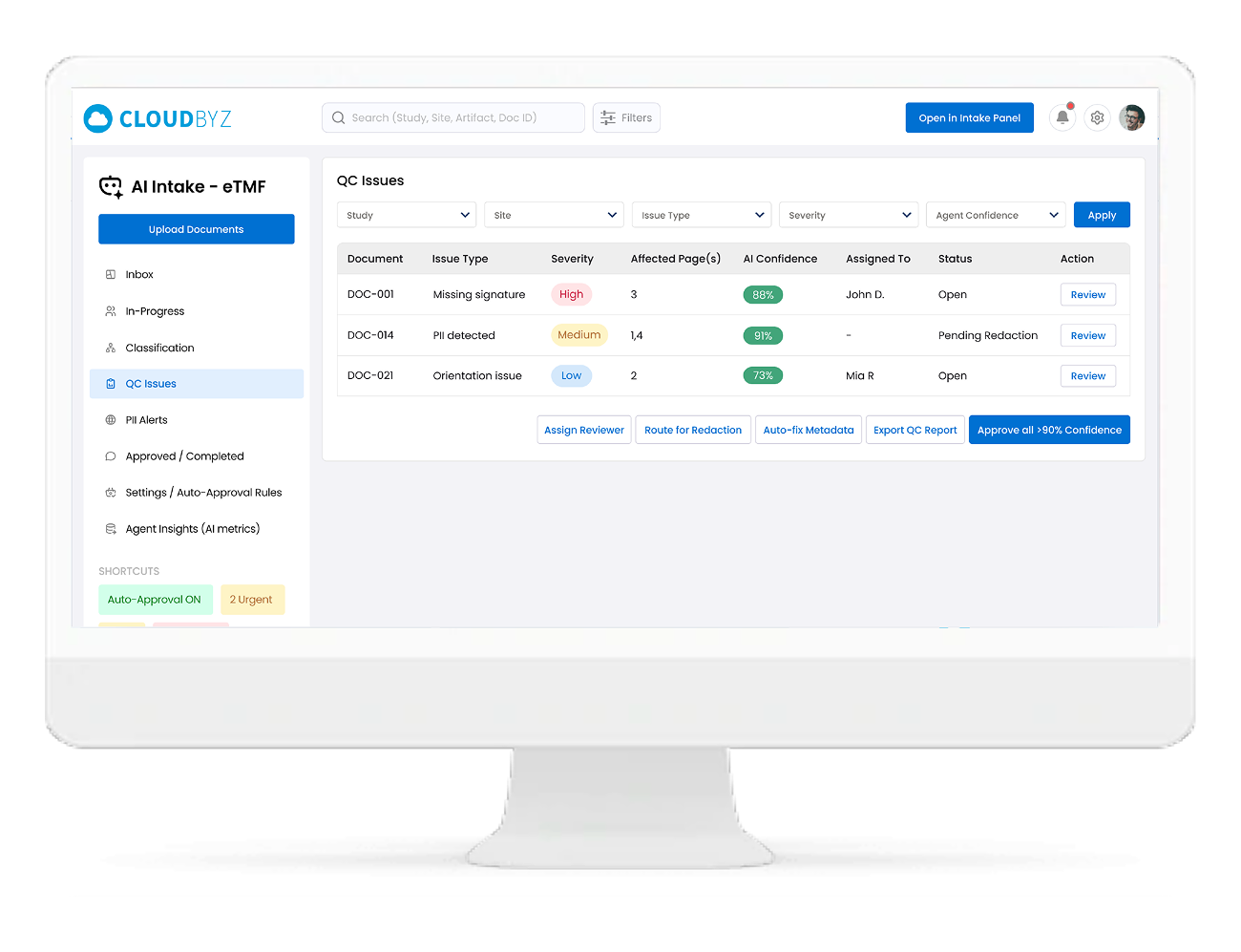
Built-In Quality Control & Compliance Checks
The Agent continuously checks each document against TMF quality rules, including:
-
- Completeness: Missing pages, missing signatures, missing dates
- Orientation & legibility: Rotated pages, blurry scans, unreadable sections
- ALCOA(+): Attributable, Legible, Contemporaneous, Original, Accurate
- Metadata validation: Does the content match the assigned study, site, artifact, and version
Low confidence or failed checks are sent to a QC Issues queue, with clear reasons and suggested fixes.
.png)
Built-In Quality Control & Compliance Checks
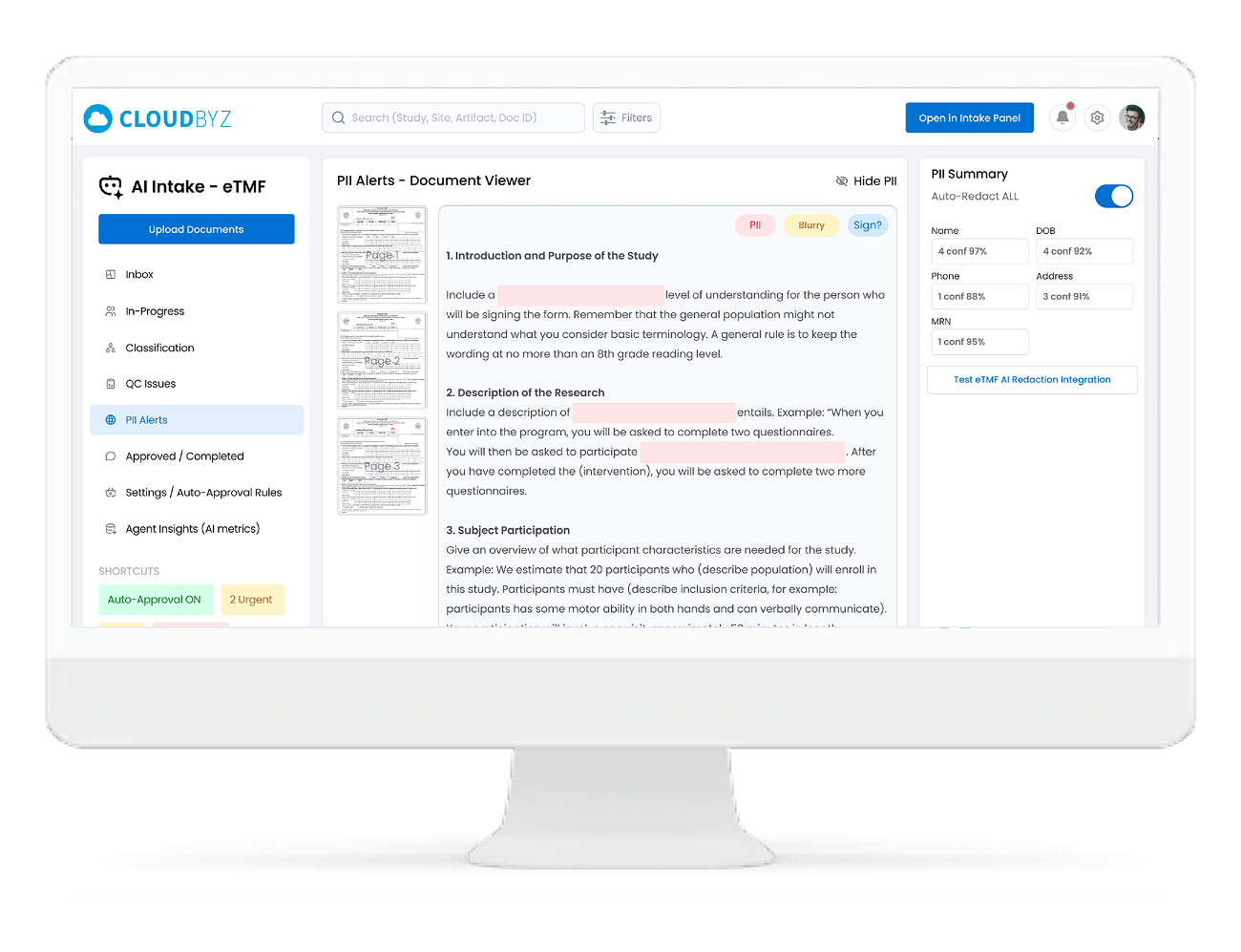
PII/PHI Detection & Redaction Routing
PII/PHI Detection & Redaction Routing
-
- Automatically detects PII/PHI (names, DOB, addresses, MRNs, emails, phone numbers, signatures, etc.)
- Flags documents requiring redaction before external sharing
- Routes sensitive files to a Redaction queue (and can integrate with your redaction tool/agent)
- Maintains a full audit trail of what was redacted, when, and by whom
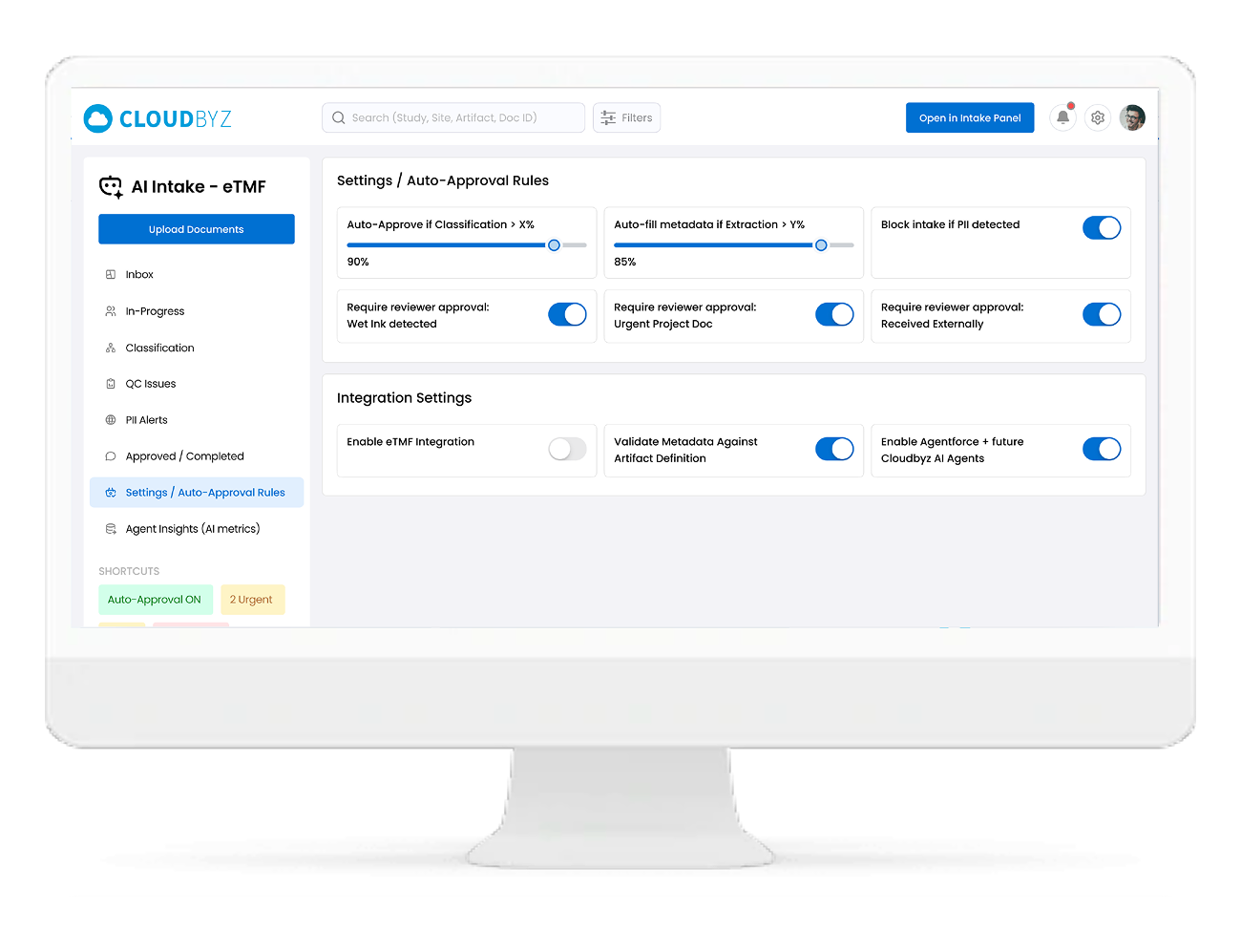
Configurable Auto-Approval Rules
You stay in control of how much you trust the AI.
-
- Define confidence thresholds for auto-classification and metadata acceptance
- Configure rules like:
- “Auto-approve IMP shipping docs with confidence ≥ 95% and complete metadata”
- “Always send informed consent forms to manual review”
- Safe defaults with easy tuning as your team gains confidence in the Agent
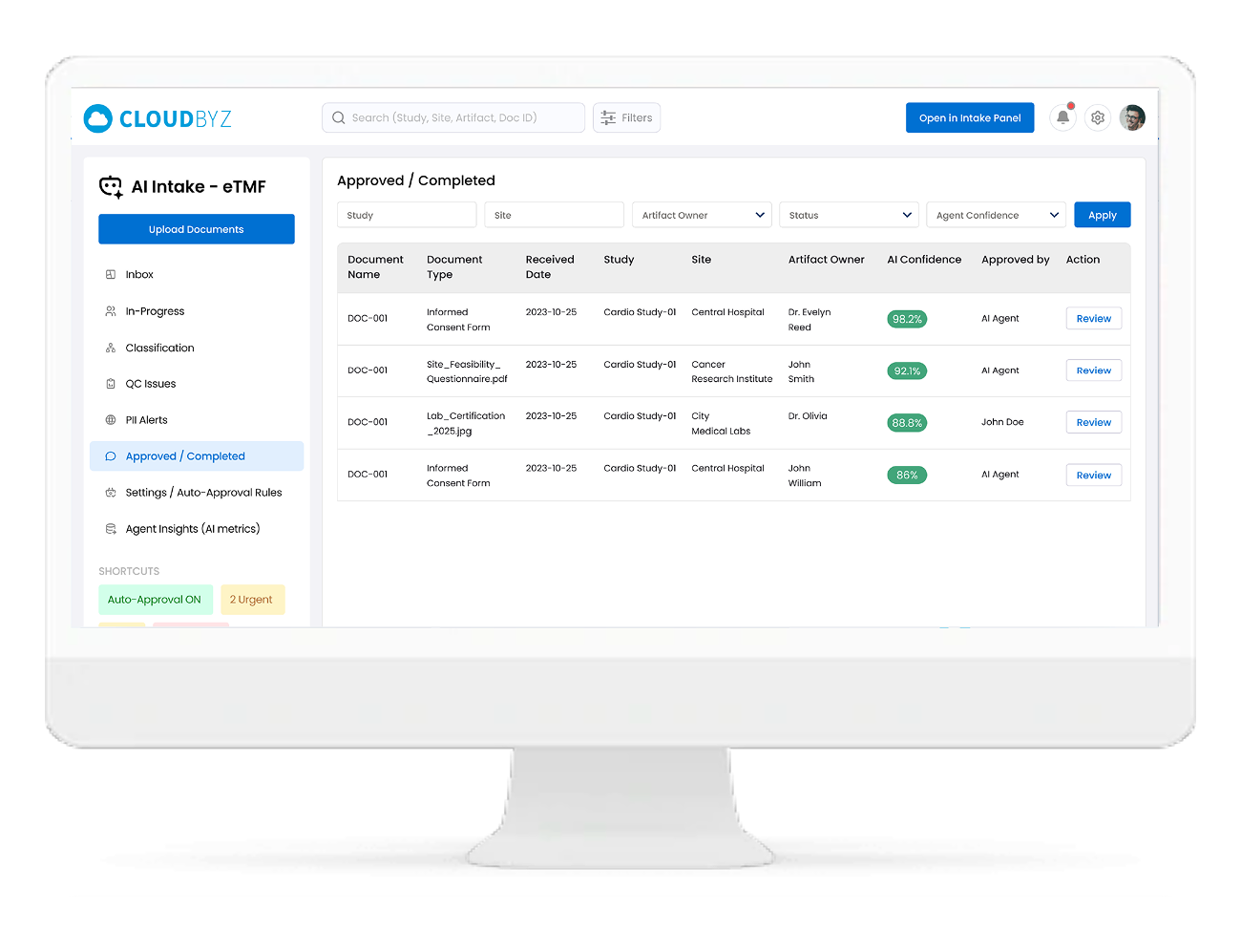
Configurable Auto-Approval Rules
Reviewer Workspaces & Consolidated QC Lists
Reviewer Workspaces & Consolidated QC Lists
-
- Reviewer workspace with a prioritized queue of documents needing attention
- Consolidated view of QC issues across studies, sites, and artifact types
- Inline side-by-side view: document preview, AI suggestions, and metadata grid
- One-click accept/override for classification and metadata
Integration-Ready with Your eTMF & Future AI Agents
-
- Designed to sync with your eTMF repository (e.g., Veeva Vault, Cloudbyz eTMF, or other platforms via API)
- Pushes final, approved documents with clean metadata and QC status
- Ready to interoperate with future Vault or platform AI agents as they become available
- Open APIs and event-based architecture to plug into your broader eClinical ecosystem
Integration-Ready with Your eTMF & Future AI Agents
.png)
Integration-Ready with Your eTMF & Future AI Agents
How It Works – From Upload to eTMF
The AI eTMF Agent is a smart intake and quality assistant that sits in front of your eTMF. It automates document routing, classification,
metadata extraction, QC, and PII redaction—while keeping humans in control for final decisions.
Think of it as a dedicated digital TMF intake specialist that works 24/7, never gets tired, and documents everything it does.
Upload & Context
User uploads single or bulk documents and provides minimal context (e.g., study, site, document type hint).
AI Analysis & Draft Suggestions
The Agent runs OCR (if needed), classifies the file, extracts key metadata, checks quality, and identifies PII/PHI.
Human Review (When Needed)
TMF Intake Agents see AI suggestions with confidence scores, quickly accept or adjust classification and metadata, and resolve QC flags.
Redaction & Final QC
Documents requiring redaction or deeper QC are routed to specialized queues and processed accordingly.
Sync to eTMF
Once approved, the document is synced to your eTMF with complete, validated metadata and a full audit trail of AI and human actions.
Upload & Context
User uploads single or bulk documents and provides minimal context (e.g., study, site, document type hint).
Human Review (When Needed)
TMF Intake Agents see AI suggestions with confidence scores, quickly accept or adjust classification and metadata, and resolve QC flags.
Sync to eTMF
Once approved, the document is synced to your eTMF with complete, validated metadata and a full audit trail of AI and human actions.
AI Analysis & Draft Suggestions
The Agent runs OCR (if needed), classifies the file, extracts key metadata, checks quality, and identifies PII/PHI.
Redaction & Final QC
Documents requiring redaction or deeper QC are routed to specialized queues and processed accordingly.
Built for TMF & Clinical Operations Teams
TMF Managers
- Ensure TMF completeness and quality across all studies
Standardize intake and QC processes globally
- Reduce last-minute fire drills before inspections
Clinical Operations & Study Teams
- Faster document turnaround and filing
- Clear visibility into what’s missing, what’s pending, and what’s approved
- Confidence that critical docs are never stuck in inboxes
Quality & Compliance
- Transparent audit trail of AI and human decisions
- Configurable rules aligned with your SOPs and regulatory expectations
- Strong controls around PII/PHI handling and redaction
Measurable Outcomes
With AI eTMF Agent, customers typically aim for:
50–70%
reduction in manual metadata entry time
30–50%
faster document intake and filing cycle times
Significant reduction
in misfiled artifacts and missing metadata
Stronger inspection readiness
with QC and redaction built into daily operations
Security, Auditability & Compliance
.png)
Scale TMF Operations and Deliver Higher Sponsor Satisfaction
CROs manage high document volumes, aggressive timelines, and complex multi-sponsor workflows. The AI eTMF Agent helps CROs modernize TMF operations with automation that reduces cost, strengthens compliance, and improves delivery performance.
How CROs benefit:
- Accelerate startup and ongoing filing across multiple studies
- Standardize intake workflows regardless of sponsor or site variability
- Increase delivery speed with automated classification, metadata extraction, and QC
- Reduce TMF processing costs and minimize reliance on offshore teams
- Improve sponsor trust with real-time completeness and QC dashboards
- Reduce inspection findings with continuous surveillance and AI-driven quality checks
Outcomes for CROs:
- ✓ Faster cycle times
- ✓ Higher quality customer deliverables
- ✓ Stronger margins
- ✓ Competitive differentiation during RFPs and bid defense meetings
Ensure Oversight, Quality, and Inspection Readiness Across All Studies
Sponsors face the challenge of overseeing multiple CROs, partners, and global sites—each with different processes and document quality levels. The AI eTMF Agent enables sponsors to centralize quality, streamline intake, and ensure consistency across the entire study portfolio.
How Sponsors benefit:
- Standardize filing and metadata requirements across CROs, sites, and regions
- Identify TMF issues earlier with automated QC and risk flags
- Improve oversight with real-time dashboards for completeness, timeliness, and quality
- Reduce study delays due to misfiled or missing artifacts
- Strengthen regulatory confidence with always-ready TMF health indicators
- Lower audit/inspection risk by ensuring continuous adherence to ALCOA+
Outcomes for Sponsors:
- ✓ Unified TMF operations
- ✓ Reduced CRO management overhead
- ✓ Fewer late-phase TMF surprises
- ✓ Faster study closure and regulatory submissions
